Zhengzhou ChangHeYue New Material CO.,LTd
Introduction to Sulfur in Petroleum Coke
Petroleum coke (petcoke), a critical raw material in carbon product manufacturing, is categorized by sulfur content into low-sulfur (<2%), mid-sulfur (2–4%), and high-sulfur (>4%) grades. Traditionally, low-sulfur coke dominated carbon production due to its stability. However, dwindling low-sulfur coke supplies have forced industries to adopt mid- and high-sulfur alternatives. This shift introduces challenges: sulfur volatilization during high-temperature calcination (above 1,500°C) significantly impacts coke’s microstructure, graphitization efficiency, and final product quality.
Key challenges include:
- Crystal expansion: Inorganic sulfur release above 2,400°C induces microcracks in graphite products.
- Density limitations: Sulfur content >4% reduces true density of calcined coke.
- Resistivity risks: Excessive sulfur increases porosity and electrical resistivity.
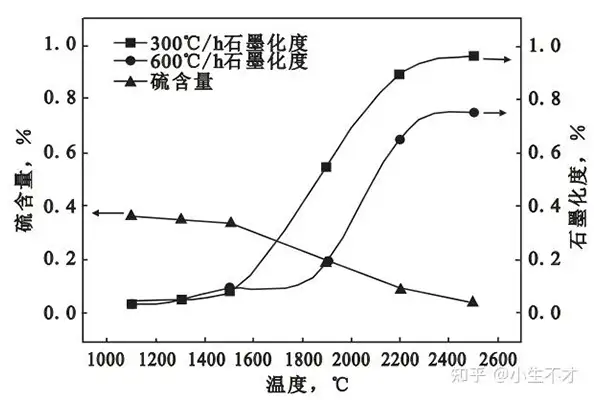
Sulfur Release Dynamics and Graphitization
Temperature-Dependent Sulfur Volatilization
- Below 1,500°C: Minimal sulfur release and no graphitization.
- 1,500–2,200°C: Rapid sulfur volatilization triggers structural transformation:
- Pore development accelerates.
- Crystallite layer spacing decreases (d002).
- Graphitization degree surges due to sulfur’s catalytic gasification.
- Above 2,200°C: Sulfur release slows, graphitization plateaus. Residual sulfur drops to trace levels at 2,500°C.
Critical Insight: Optimizing sulfur’s thermodynamic release kinetics (e.g., controlled heating rates) can accelerate graphitization without inducing structural defects.
Impact of Sulfur Release on True Density
Experimental data reveals a direct correlation between sulfur volatilization and true density:
- <1,500°C: Slow sulfur release → density increases marginally (0.05–0.10 g/cm³).
- >1,500°C: Accelerated sulfur escape → density rises sharply (up to 0.30 g/cm³).
Mechanism: Sulfur removal reduces pore-forming impurities, densifying the coke matrix. High-sulfur coke (>4%) requires precise temperature control to balance density gains and crack risks.
Optimizing Graphitization: Strategies for High-Sulfur Coke
- Controlled Heating Rates
Avoid excessive ramp-up speeds (>50°C/min) to prevent uneven sulfur release and microcracking. - Multi-Stage Calcination
- Stage 1: 800–1,200°C (organic sulfur removal).
- Stage 2: 1,500–2,200°C (inorganic sulfur release + graphitization).
- Stage 3: 2,300–2,500°C (final densification).
- Additive Catalysts
Iron or boron compounds can lower sulfur’s activation energy, enabling efficient removal at lower temperatures. - Post-Calcination Treatments
Mechanical compression or chemical vapor infiltration (CVI) to mitigate porosity.
Conclusion
High-temperature sulfur release profoundly shapes petroleum coke’s graphitization efficiency and product quality. Key takeaways:
- Sulfur volatilization above 1,500°C drives graphitization but requires kinetic control.
- True density improvements correlate with sulfur removal rates.
- Strategic thermal profiling and catalytic additives minimize defects in high-sulfur coke processing.
For industries adopting mid/high-sulfur coke, optimizing calcination parameters is essential to balance cost, performance, and sustainability.